NICE, France - November 15, 2011 - Iris Pharma, a world renowned contract research organisation specialising in preclinical and clinical ophthalmology research, has announced that it has just enhanced its equipment pool by acquiring a Spectralis®HRA+OCT and a Luminex®Lx200 IS analysis system.
As one of the few contract research organisations in the world to benefit from owning these two items of equipment, Iris Pharma has thus further sharpened its expertise in terms of preclinical ocular research and can offer its customers very high precision evaluation tools for monitoring treatments during studies. According to Philippe Margaron, Director of the Preclinical Department, "being able to observe and measure the smallest anatomical or biochemical change and the evolution of the disease in an animal model in total confidence can greatly influence the decisions taken in respect of a new treatment". In addition, the benefit of the Spectralis®HRA+OCT is that continuity can be established between the preclinical and clinical research, as it can be used on humans and on animals at the same time: "the study of the animal imitates the treatment of a patient, and gives our customers access to the same techniques as those available to doctors".
Imaging and research benefit
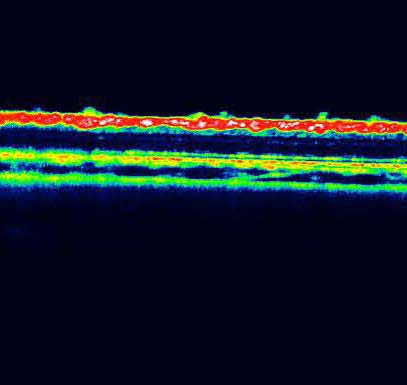
The Spectralis®HRA+OCT allows very high precision monitoring of the areas of the retina which have been treated and their evolution. This multimodal imaging technique offers an in-depth view of the structure and an overall picture of the damages in real time, and consequently plays a major role in the detection of insults to the retina and the pigmentary epithelium. With the Luminex Lx200 analysis system, biochemical quantifications and multiple simultaneous analyses can be carried out in low sample volumes. Iris Pharma is already able to undertake analyses of cytokines in models of inflammations, and in particular in the model of EIU (endotoxin-induced uveitis).
Imaging and ethics
Non-invasive imaging helps in the application of the 3 Rs principle (Replacement, Refinement, Reduction) in laboratory animal welfare. It allows sections of tissue to be visualized in vivo, and hence the evaluation of the disease and its evolution while the animal is alive. By increasing the number of markers and combined analyses, a larger amount of information is therefore obtained from a single individual. Moreover the reliability of machines of this kind means better data can be obtained: the need for complementary or confirming studies is minimized.
About Iris Pharma:
Iris Pharma is a French independent CRO that specializes in preclinical and clinical research in the field of ophthalmology. Founded in 1989 by Dr Pierre-Paul Elena, Iris Pharma offers its expertise in the development of ophthalmic drugs and ocular medical devices to the pharmaceutical industry and biotechnology companies around the world. Iris Pharma services include: proof of concept and GLP preclinical studies (tolerance, PK, efficacy), bioanalysis, preclinical formulation, clinical trials and consulting services. For more information, see: https://www.iris-pharma.com
NICE, FRANCE - 15 novembre 2011 - Iris Pharma, société de recherche sous contrat (CRO) de renommée mondiale spécialisée dans la recherche préclinique et clinique en ophtalmologie, a annoncé qu'elle venait d'étoffer son parc d'appareils en acquérant un HRA-OCT Spectralis® et un système d'analyse Luminex® Lx200 IS.
Comptant parmi l'une des rares CRO au monde à bénéficier de ces deux appareils, Iris Pharma aiguise encore ainsi son expertise en matière de recherche préclinique oculaire et propose à ses clients des outils d'évaluation de très grande précision pour le suivi des traitements pendant les études. Selon Philippe Margaron, directeur du département préclinique, "pouvoir observer et mesurer en toute confiance le moindre petit changement anatomique ou biochimique et l'évolution de la maladie dans un modèle animal peut influer grandement sur les décisions prises vis-à-vis d'un nouveau traitement". De plus, l'OCT Spectralis® a pour avantage d'établir une continuité entre la recherche préclinique et clinique car à la fois utilisé chez l'homme et chez l'animal : "l'étude sur l'animal imite le traitement d'un patient et donne accès à nos clients aux mêmes techniques que celles à disposition des médecins".
Imagerie et bénéfice recherche
Le HRA-OCT Spectralis® permet de suivre avec une très grande précision les zones traitées de la rétine et leur évolution. Cette technique d'imagerie multimodale offre une vision profonde de la structure et un portrait global de l'affection en temps réel et joue, par conséquent, un rôle majeur dans la détection des atteintes rétiniennes et de l'épithélium pigmentaire. Le système d'analyse Luminex Lx200, quant à lui, permet d'effectuer des quantifications biochimiques et de multiples dosages simultanés à partir de faibles volumes d'échantillons. Il permet déjà à Iris Pharma de réaliser des dosages de cytokines dans ses modèles d'inflammation et notamment dans le modèle de l'EIU (Endotoxin-induced uveitis).
Imagerie et éthique
L'imagerie non-invasive aide à mettre en application le principe des 3 Rs (Replacement, Refinement, Reduction). Elle permet de réaliser des coupes in vivo et des évaluations de la maladie et de son évolution du vivant de l'animal, et, en augmentant le nombre de marqueurs et de mesures combinées, permet d'augmenter le nombre d'informations obtenues à partir d'un seul individu. Par ailleurs la fiabilité de telles machines permet d'obtenir de meilleures données : ceci limite le besoin de recourir à des études complémentaires ou de réitérer les études.
A propos d'Iris Pharma :
Iris Pharma est une société française de recherche sous contrat (CRO) indépendante spécialisée dans la recherche préclinique et clinique en ophtalmologie. Fondée en 1989 par le Dr Pierre-Paul Elena, Iris Pharma propose son expérience et son savoir-faire dans le développement de médicaments ophtalmologiques et de dispositifs médicaux oculaires aux industries pharmaceutiques et aux sociétés de biotechnologie à l'échelle mondiale. Les services offerts par Iris Pharma regroupent les études précliniques BPL ou non-BPL (tolérance, PK, efficacité), la bio-analyse, la formulation préclinique, les essais cliniques ainsi que le conseil stratégique.
Pour plus d'information voir : https://www.iris-pharma.com