Pharmacokinetic (PK) and toxicokinetic (TK) analyses provide information that can guide the selection of dose levels and administration frequency for future animal and clinical studies. Iris Pharma offers complete ocular PK and TK evaluation services in a Good Laboratory Practice (GLP) environment, which allows us to obtain reliable data that is required before first-in-human and phase I clinical studies of your product.
Iris Pharma performs ocular pharmacokinetics studies, including:
Formulation: Eyedrop, eyegel, ointment, insert, particle, implant, patch, microspheres, cream, …
Administration: Topical ocular application, intravitreal injection (IVT), subconjunctival injection, subTenon injection (SBT), retrobulbar injection, periocular injection, intracameral injection, subretinal injection, intrascleral, transscleral, intrastromal, intravenous, intraperitoneal, intramuscular, iontophoresis …
Animals: Rabbits, rats, guinea-pigs, mice (albino and pigmented)
Sampling:
Detection:
Parameters: Tmax, Cmax, AUC, % of applied dose, t½
Toxicokinetic endpoints are similar to those used for pharmacokinetics, except that samples are collected during toxicology and safety studies. These results help us understand a drug's behavior at the maximal dose used in toxicology studies, and provide steady state, accumulation, and trough levels after repeated administration.
Iris Pharma has been a Good Laboratory Practice (GLP)-compliant bioanalytical laboratory since 1995, and we conduct validations and study sample analyses that meet recognized international guidelines, including those issued by the United States Food and Drug Administration (FDA), the International Conference on Harmonisation (ICH), and the Organization for Economic Co-operation and Development (OECD).
Iris Pharma develops, customizes, and validates assays of drug candidates and metabolites in a variety of ocular matrices to support preclinical, biopharmaceutical, and clinical pharmacology programs.
We are experts in processing samples using tools that are both fast and sensitive, such as:

Iris Pharma offers routine bioanalytical testing in multiple biological species and rare ocular matrices.
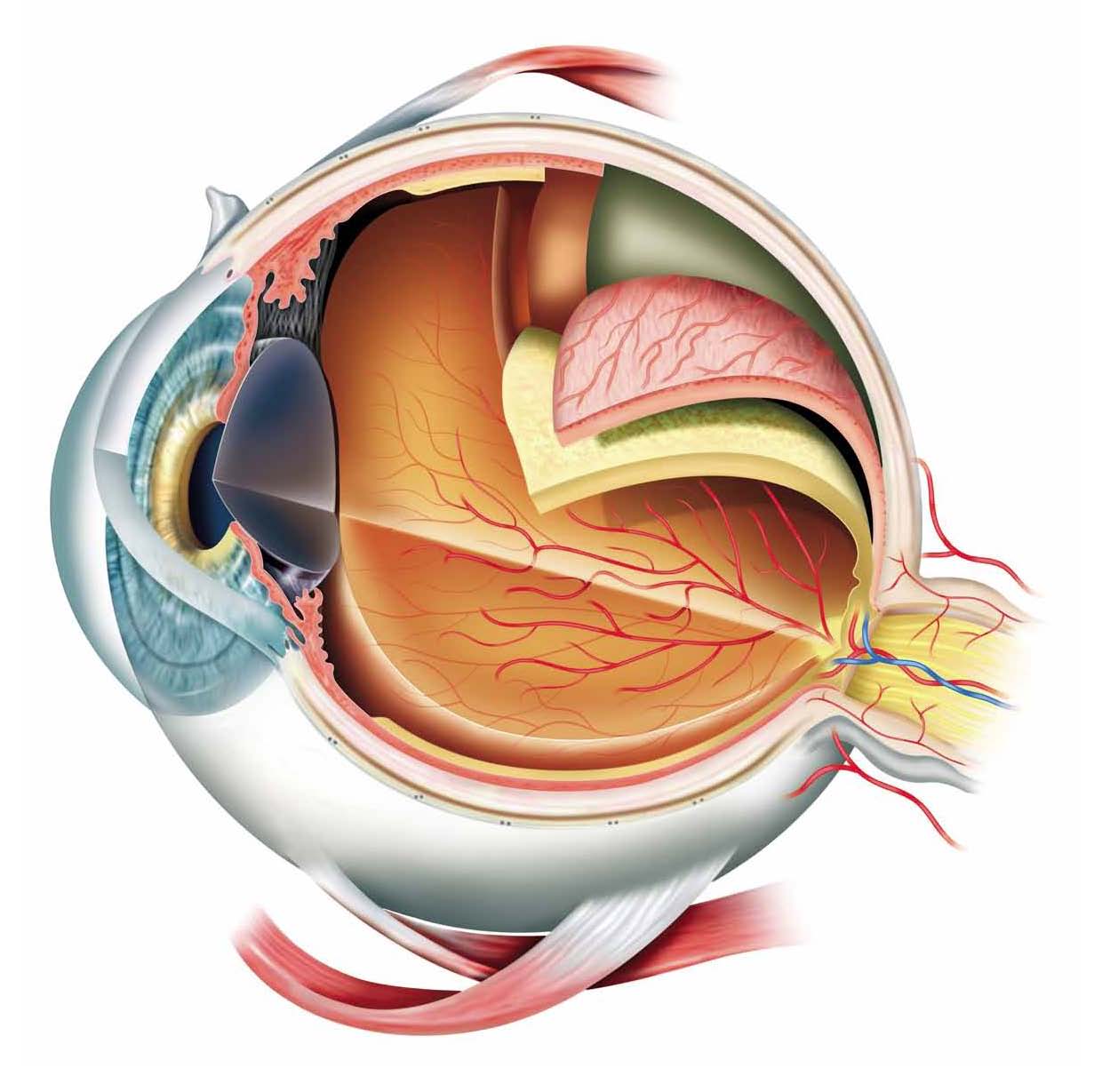
The primary matrices used at Iris Pharma include:
Tears |
Ciliary body
|
Palpebral Conjunctiva |
Vitreous |
Bulbar Conjunctiva
|
Retina |
Aqueous Humor
|
Choroid |
Cornea
|
Sclera |
Lens |
Optic nerve |
Iris |
Plasma and Blood
|
We are experts in processing samples using tools that are both fast and sensitive, such as:
